Chemfort protects against bacterial contamination for 7 days
The National Institute for Occupational Safety and Health (NIOSH) defines a closed-system drug transfer device (CSTD) as “a drug transfer device that mechanically prohibits the transfer of environmental contaminants into the system and the escape of the hazardous drug or vapor concentrations outside the system.”(1)
CSTDs are frequently used in preparation and administration of oncology drugs. Cancer patients are often immunosuppressed and therefore vulnerable to developing infections, which can progress rapidly. The risk of bacterial infection in such patients can include recurring pneumonia, bronchitis, dysfunction,(2) and even mortality (60%).(3) Thus, it is vital that drugs prepared and administered to oncology patients are sterile and clean from any bacteria.
The FDA recommends the evaluation of the ability of a medical device to resist or inhibit the transfer of infectious microorganisms under repeated simulated use conditions(4) in a test referred to as microbial ingress. The FDA has provided guidance concerning the practice of microbial ingress testing. The test should demonstrate that a disinfected device would not transmit bacteria, approving a device for the usage period and number of connection/disconnection cycles included in its labeling. The Chemfort® CSTD was tested in light of the FDA guidance, and the results were part of the system’s premarket 510(k) clearance under the ONB product code (reserved for closed antineoplastic and hazardous drug reconstitution and transfer systems).
Study outlines
The study aim was to confirm the efficacy of Chemfort® closed system components in preventing the passage of bacteria through the devices for up to 7 days and 10 connection/disconnection cycles (activations).
Method
The four selected bacterial species include representatives from both classes of bacterial species (Gram-negative and positive). Some other criteria for their selection were high motility and frequent association with hospital-acquired infections.(5)
Chemfort® Vial Adaptors (VAs) were applied on sterile glass vials containing 100 mL saline (Aminolab Ltd., Ness Ziona, Israel), and inoculated with one of the four types of bacteria. Disposable syringes with male Luer lock connections were attached to Chemfort® Syringe Adaptors (SAs), and the SAs were connected to Chemfort® VAs after both septa were disinfected using either a 70% isopropyl alcohol (IPA) pad or a 70% IPA swab stick. During each activation, 3 mL of saline were withdrawn from each vial to the connected syringe. Then the SAs were disconnected from the VAs, and both were immediately capped until the next activation. Following disconnection between the VA and SA, the SA was disconnected from the syringe and the saline was passed through a sterile filter with a 0.45 μm membrane. The syringe was immediately reconnected to the SA and capped. The membranes were washed with 0.1% Tween and placed on petri dishes containing agar and incubated at 30-35ºC for 2-5 days. For each device, this procedure, beginning with application of additional microbes to the septa, was repeated a total of 10 times over the course of 7 days (168 hours).
For the positive control group, the same procedure was conducted, excluding disinfection. For the negative control group, the same disinfection, activation, and withdrawal procedures were conducted, but without inoculation.
For the bacterial lethality group, VAs (not connected to vials) were inoculated with test bacteria, transferred into a sterile bag containing 100 mL saline with 0.1% Tween 80, and shaken for 15 minutes, assuring maximum agitation. Part of the extract was filtered and incubated in the same fashion as for the test groups. This procedure was repeated 10 times (with fresh VAs each time) in parallel to the 10 activations for the other test and control groups.
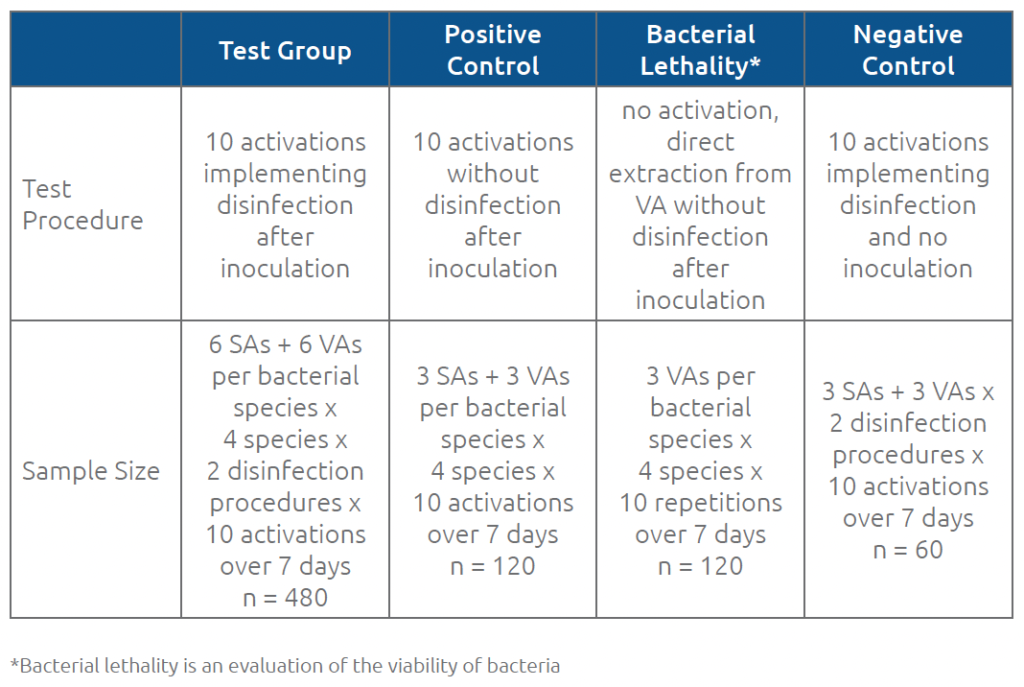
For all test and control samples, the total number of colonies on each dish was counted and recorded. Table 1 summarizes the procedures and sample sizes for each group.
Table 1. Procedures and sample sizes for test and control groups
Results & Discussion
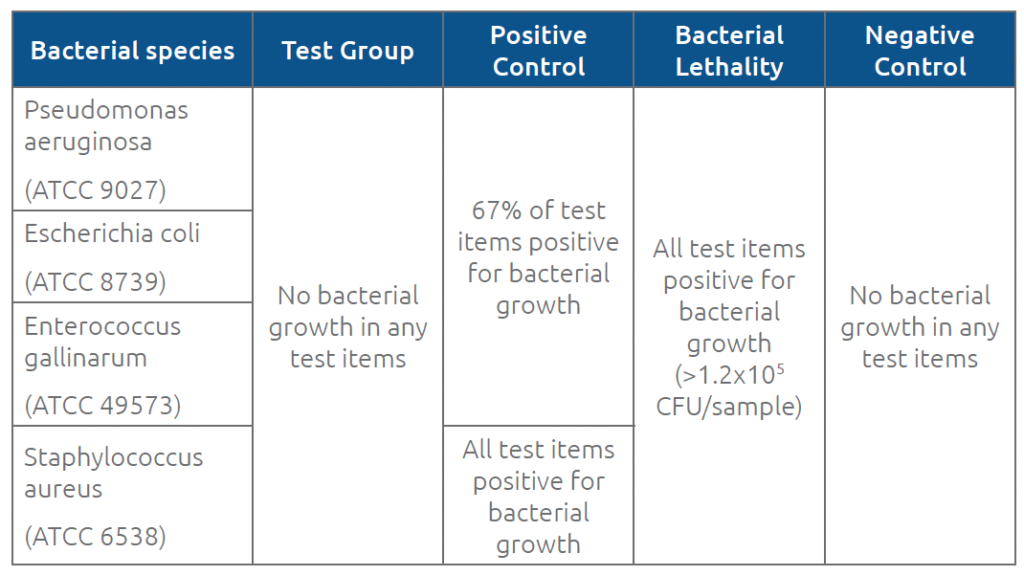
Acceptance criteria were defined as <1 colony forming unit (CFU)/sample (no microbial growth) for test items and negative controls, >1 CFU/sample for positive control items (detectable microbial growth), and > 103 CFU/sample (extensive microbial growth) for bacterial lethality items.
No signs of microbial growth were observed in any of the test group items during the 7-day test period including 10 activations, when using the recommended disinfection procedures (70% IPA swab sticks or 70% IPA pads). For the positive control group, at least one item tested with each of the 4 bacterial species at each activation showed microbial growth. The fact that some positive control samples (no disinfection) were negative for growth indicates that the Chemfort® septa sometimes prevent ingress even when disinfection is neglected. To obtain a more reliable indication of bacterial viability during the test, the bacterial lethality test was performed, which didn’t require bacterial to penetrate the Chemfort® septa in order to be detected. For the bacterial lethality group results, all items tested showed more than 103 CFU per vial connector. Table 2 summarizes the results for each group.
Table 2. Results for test and control groups
The fact that no growth was observed in any of the test items throughout their 7-day usage period and maximum number of connection/disconnection cycles demonstrates Chemfort®’s ability to maintain microbiological integrity even under highly challenging conditions. These results are in alignment with other studies testing Chemfort®’s ability to maintain sterility even beyond the regulatorily approved usage period of 7 days(6) as well as the system’s ability to prevent viral ingress.(7) The successful performance of Chemfort® in the microbial ingress test supports its definition as a CSTD according to NIOSH and aided its FDA-clearance under the ONB product code. It also allows potential cost savings by allowing drugs that are physically and chemically stable for at least 7 days to be used for 7 full days (168 h), when they might otherwise be discarded due to concerns of microbial contamination.(8)
References
- NIOSH Alert: Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings 2004. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No 2004–165.
- Safdar A, Bodey G, Armstrong D. (2011) Infections in Patients with Cancer: Overview. Principles and Practice of Cancer Infectious Diseases, 3–15. https://doi.org/10.1007/978-1-60761-644-3_1
- Bhat S, Muthunatarajan S, Mulki SS, Archana Bhat K, Kotian KH (2021). Bacterial Infection among Cancer Patients: Analysis of Isolates and Antibiotic Sensitivity Pattern. International journal of microbiology, 8883700. https://doi.org/10.1155/2021/8883700
- Guidance for Industry and FDA Staff: Intravascular Administration Sets Premarket Notification Submissions [510(k)], U.S. Department of Health and Human Services Food and Drug Administration, 2008, Rockville, MD. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm070850.pdf
- Pechersky L. (2019). Microbial Ingress Testing of Tevadaptor^2 Closed System Components: Syringe Adaptor and Vial Adaptor (Report No. 5611). Data on file at Simplivia.
- Walker KE, Machníková R, Ozolina L, Wilkinson AS, Johnson AJ. (2022) Eur. J. Hosp. Pharm. Published Online First: doi: 10.1136/ ejhpharm-2021-003148
- Amichay T, Shimon O, Raveh E. (2021) Pharm. Pract. 19(4): 2576.
- Juhasz A, Batka G, Szucs A. (2016). Drugs and Therapy Perspectives 32(4):170-6.
For more information: open the PDF document