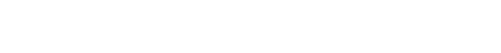Automating Chemotherapy,
Elevating Patient Care
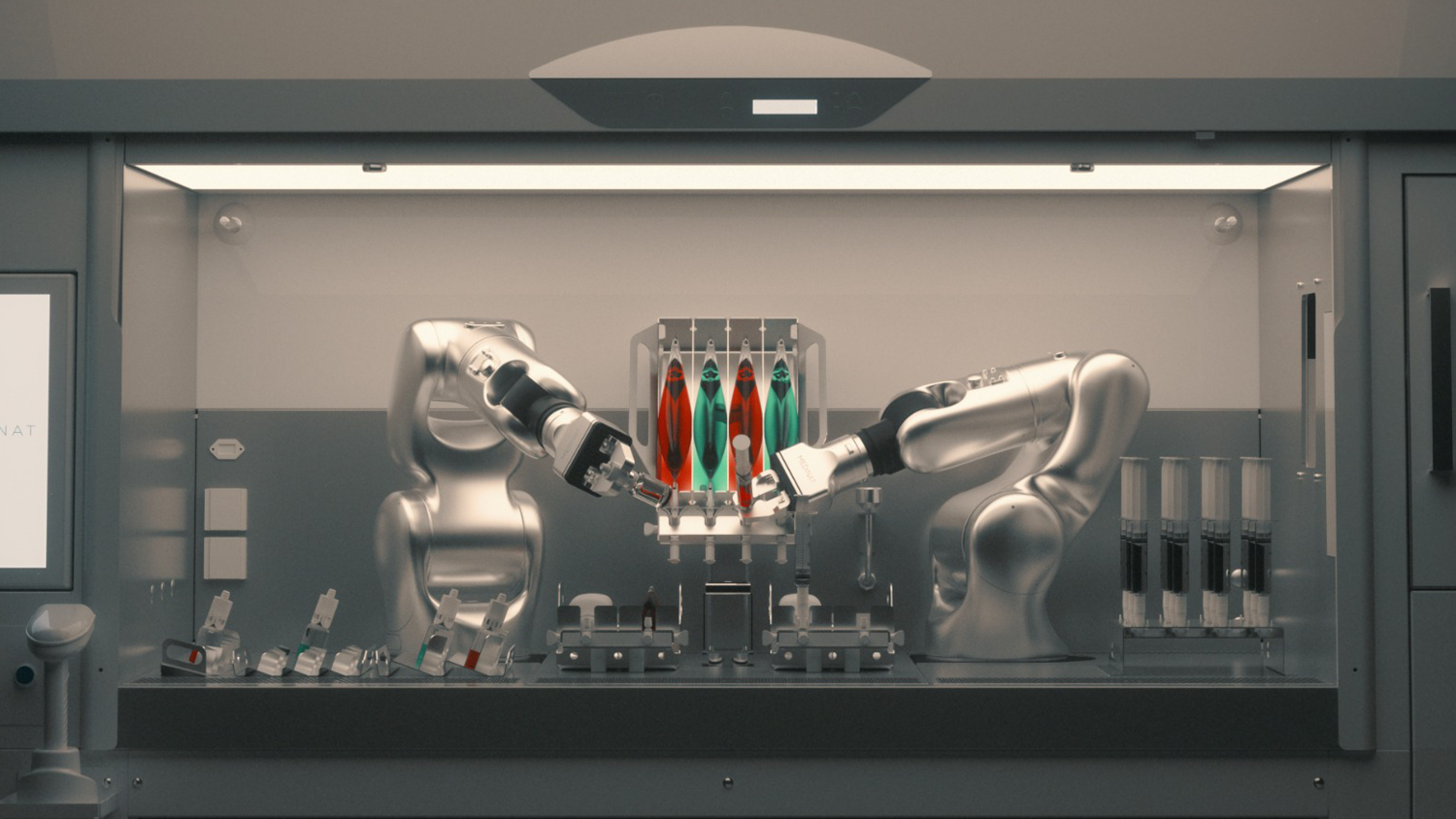
IV ICON Cyto is a cutting-edge robotic system designed to automate and optimize the preparation of personalized chemotherapy drugs. The system and the automated preparation process comply with the most stringent quality standards for the preparation of sterile injectable drugs (GMP ANNEX 1 COMPLIANCE), ensuring the highest level of safety and quality.
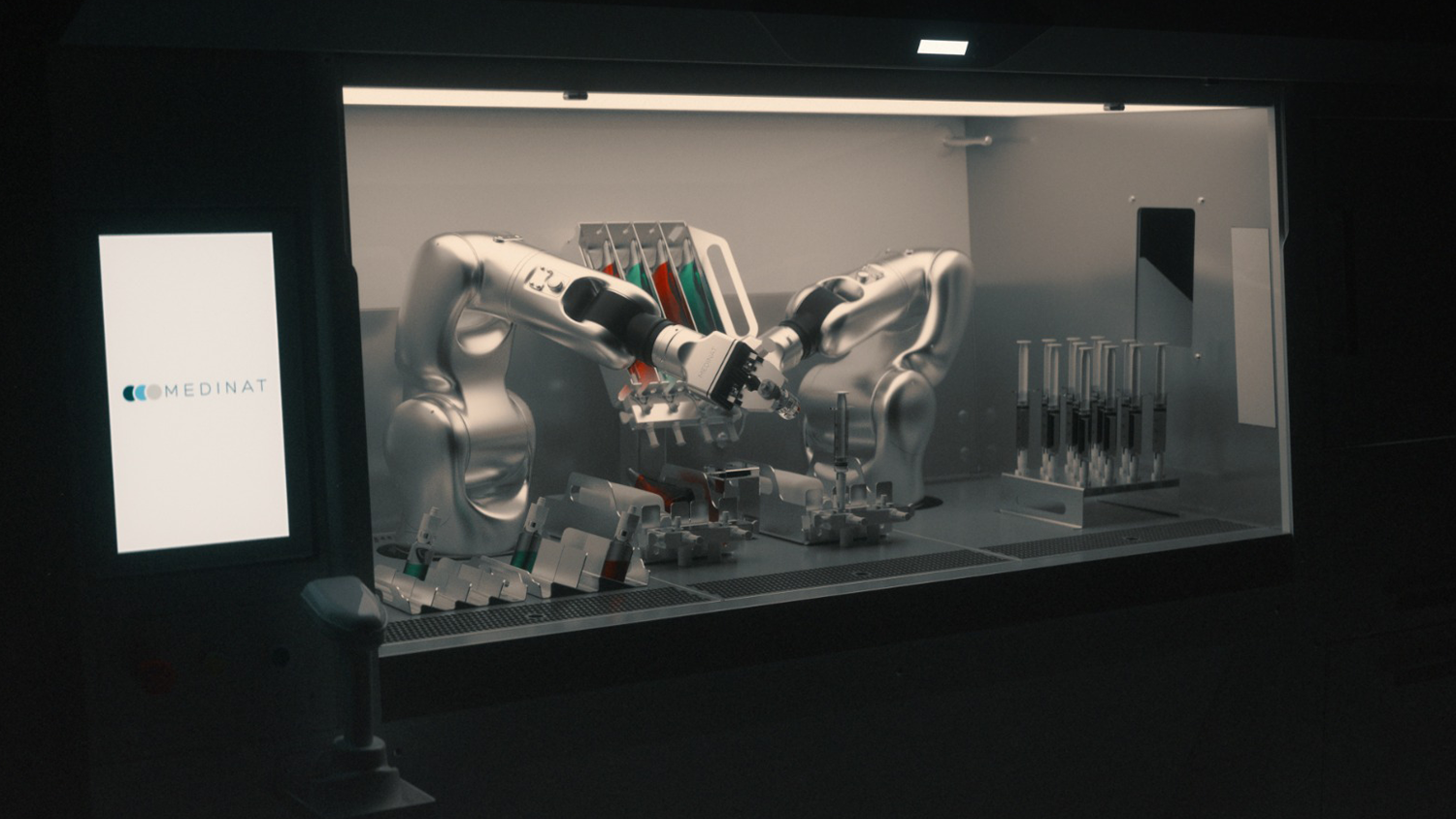
Thanks to the perfect synergy between the two robotic arms, IV ICON Cyto ensures an efficient and fully traceable process, improving the accuracy and transparency of operations.

Efficiency and Safety
IV ICON Cyto automation reduces human error and increases dosing accuracy, significantly improving treatment quality and ensuring compliance with GMP standards for sterile drug preparation.
The automated system monitors and verifies each step of the process, minimizing contamination risks and optimizing cleanroom usage.
This approach allows operators to focus on higher value-added activities, while the system accurately manages repetitive and critical tasks, often subject to human error.
Intuitive Management
IV ICON Cyto features a convenient and intuitive management software that guides the operator through every step of the process, from start to finish. The user interface simplifies daily operations, ensuring that each step is performed correctly and automatically documented.
The flexible workflow integrates seamlessly into the routine of the hospital pharmacy, easily adapting to the specific requirements of each facility, ensuring a smooth and seamless process.
CSTD - The advantages of a closed system
The use of a Closed System Transfer Device (CSTD) consumable is a key safety feature. This closed system eliminates the risk of exposure to hazardous substances for personnel and prevents cross-contamination, making the process compliant with the most stringent safety standards.
CSTD adapters also improve operational efficiency, ensuring protection during all phases, from preparation to patient administration.
Technical features
Controlled Work Environment
IV ICON Cyto creates a sealed environment during the preparation process, minimizing contamination risks and ensuring compliance with GMP, ISO 14644 and EN 17141 standards.
Automated Control
The system uses gravimetric control and machine vision technology to ensure maximum precision in each dose, with real-time verification and automated traceability.
Intelligent Waste Management
The system also automates waste management, reducing risks for personnel and optimizing the operational flow.
Integration with information systems
Integration with the Hospital Information System (HIS) is guaranteed by a message exchange based on the HL7 protocol, ensuring seamless connectivity and perfect integration with existing workflows.
System requirements

IV ICON Cyto
With IV ICON Cyto, the preparation of chemotherapy drugs reaches a new level of safety, precision and automation.
GMP compliance, personnel protection and integration with hospital systems via HL7 protocol, make IV ICON Cyto the ideal solution to optimize pharmaceutical production and improve the quality of care for cancer patients.